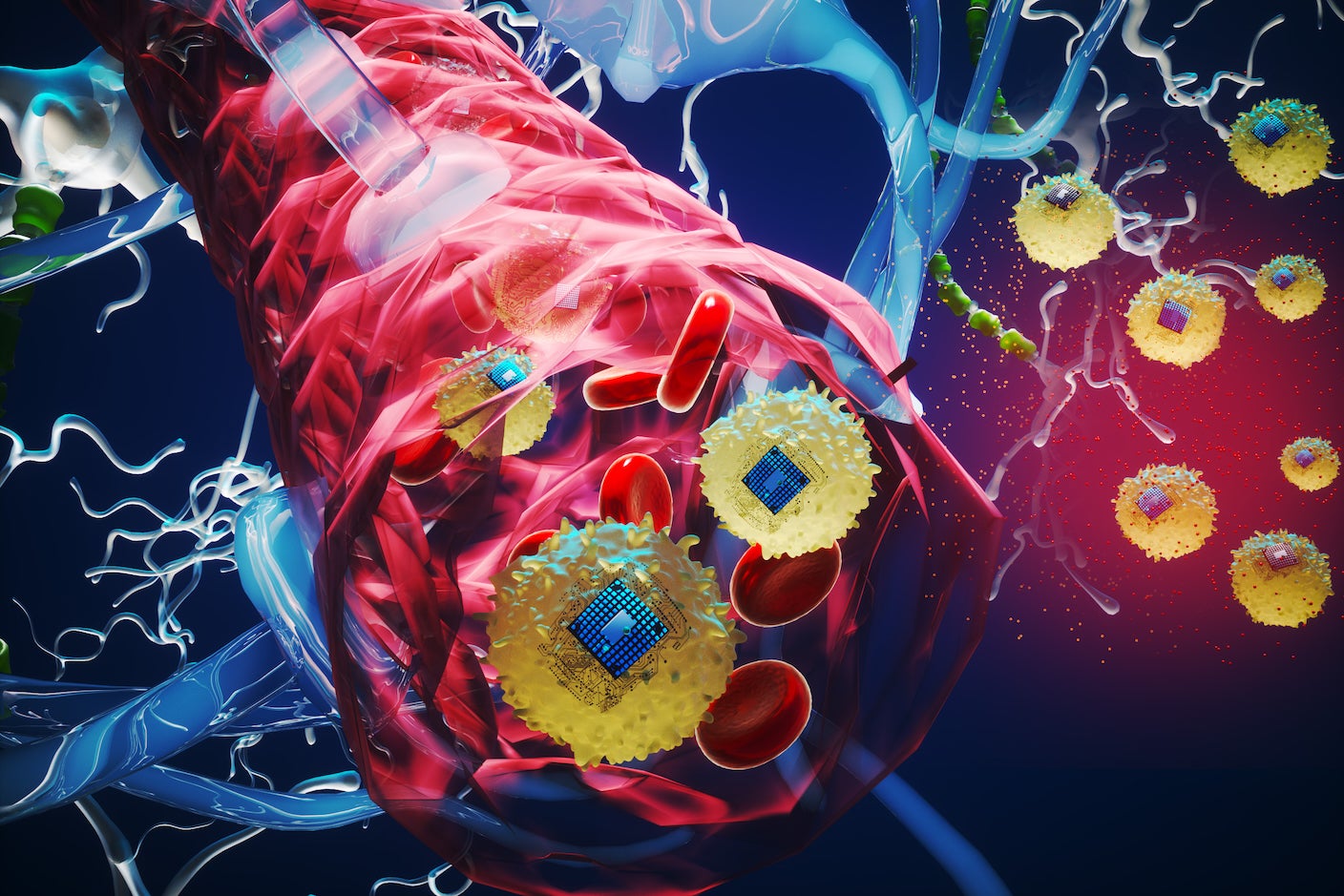
From restoring movement and speech in people with paralysis to fighting depression, brain implants have fundamentally changed lives. But inserting implants, however small or nimble, requires risky open-brain surgery. Pain, healing time, and potential infections aside, the risk limits the technology to only a handful of people. Now, scientists at MIT Media Lab and collaborators hope to bring brain implants to the masses. They’ve created a tiny electronic chip powered by near-infrared light that can generate small electrical zaps. After linking with a type of immune cell to form bio-electronic hybrid chips, a single injection into the veins of mice shuttled the devices into their brains-no surgery required. It sounds like science fiction, but the injected chips easily navigated the brain’s delicate and elaborate vessels to zero in on an inflamed site, where the microchip reliably delivered electrical pulses on demand. The chips happily cohabitated with surrounding neurons without changing the cells’ health or behavior. “Our cell-electronics hybrid fuses the versatility of electronics with the biological transport and biochemical sensing prowess of living cells,” said study author Deblina Sarkar in a press release. The strategy, which the researchers call circulatronics, could radically change brain stimulation. Targeted electrical zaps have shown early promise for treatment of a variety of brain diseases, such as Alzheimer’s, depression, and brain tumors. And because the devices can be engineered to dissolve after a certain amount of time, they could potentially collect neural signals from healthy people, providing an unprecedented look into our brain’s inner workings. A Long Road Today’s brain implants are relatively bulky and struggle to reach deep into the brain. Most use batteries, either directly inside the device or in a battery pack affixed to the skull. An ideal implant would be self-powered, controllable, and small enough to move through the smallest nooks and crannies of the brain and its vessels. A previous device, about the size of a grain of rice, used magnetic energy for power and generated electrical zaps in rodents while they actively roamed around. But because the device was controlled by magnetic fields, the setup required large and expensive hardware. Magnetic particles also tend to move in straight lines. This makes them terrible at navigating our brains serpentine vessels. Near-infrared light offers an alternative to magnetic control. The wavelength easily penetrates the skull and brain with minimal scattering, suggesting it could control devices deep in the brain. Earlier this month, a team engineered an infrared-powered implant smaller than a grain of salt that could record from or stimulate neurons in mice. Although the device still required minimal surgery to implant, it reliably captured brain signals for a year, roughly half a mouse’s lifespan. Infrared light has long been on Sarkar’s radar for an injectable brain implant. For six years, her team worked to solve multiple difficult roadblocks, eventually landing on circulatronics. Tag Team The team first had to make a chip so small it could easily flow through blood vessels without damaging them. The team turned to photovoltaic components that convert light into electricity, similar to the way solar panels work. The chips are made of organic semiconductors that are biocompatible and flexible. This makes them suitable for navigation of our squishy bodies. Each one is like a tiny, light-powered battery sandwich, with a positive and negative metallic layer and an organic polymer inner filling. Roughly 10 microns in diameter and smaller than a cell, these chips can be manufactured en masse with the same technology used to make computer chips. In tests with molds simulating the brain, the chips reliably generated electrical currents. Then there was the problem of getting the chips to their target. The brain is protected by a wall of cells called the blood-brain barrier. The barrier is extremely selective of what molecules, proteins, and other materials can enter. Electronics, no matter how small, don’t make the cut. Some studies have tried to deliberately pry open the blood-brain barrier, but even a brief opening invites pathogens and other dangerous molecules inside. The team’s solution was a cellular Trojan horse. When the brain experiences inflammation, the blood-brain barrier admits immune cells called monocytes. These cells roam the bloodstream equipped with chemical beacons to hunt down inflammatory sites. In theory, microchips could catch a ride on these cells through the blood-brain barrier without forcing it open. To link monocytes to their tiny chip, the team used a Nobel Prize-winning technology called click chemistry. Think of it as Velcro. The researchers altered the surfaces of the monocytes in such a way that they formed Velcro-like “loops.” Then they added chemical “hooks” to the chips. When these components met, they clicked into place-but were still easily detachable-to form the final implant. “The living cells camouflage the electronics so that they aren’t attacked by the body’s immune system, and they can travel seamlessly through the bloodstream. This also enables them to squeeze through the intact blood-brain barrier without the need to invasively open it,” said Sarkar. Roaming Biohybrid Bots To test their hybrid implants, the team tagged them with glow-in-the-dark trackers and injected them into the veins of mice. The critters had been given a chemical that triggered inflammation at a specific site deep in their brains. Within 72 hours, the hybrid chips self-implanted into the inflamed area, whereas electronics lacking a cellular partner were barred from the brain. On average, around 14, 000 hybrid implants latched onto the brain. The devices worked as expected. After receiving pulses of near-infrared light for 20 minutes, neurons in the implanted region spiked with electrical activity at a magnitude similar to spikes trigged by current brain implants. Neighboring neurons were undisturbed. The hybrid implants didn’t seem to affect the brain’s activity. Animals with the implant roamed around as usual. They showed no sign of changes to mood, memory, or other cognitive functions, happily sipping water and maintaining body weight for six months. Despite circulating in the blood after injection, the hybrid implants had no observable impact on other organs. Although this study focused on brain inflammation, a similar strategy could be used to shuttle brain stimulation chips into stroke sites to aid rehabilitation. The system is relatively plug-and-play. Swapping monocytes for other cell types, such as T cells or neural stem cells, could allow them to act like cellular taxis for a wide range of other diseases. The team hopes to kick off clinical trials of the technology within three years through MIT spinoff company, Cahira Technologies. “This is a platform technology and may be employed to treat multiple brain diseases and mental illnesses,” said Sarkar. “Also, this technology is not just confined to the brain but could also be extended to other parts of the body in future.”.
https://singularityhub.com/2025/11/25/brain-implants-smaller-than-cells-can-be-injected-into-veins/
Brain Implants Smaller Than Cells Can Be Injected Into Veins